The FDA (U.S. Food & Drug Administration) has partnered with academia and industry the Critical Path Initiative program to create a Guidance Document for industry-wide use proper validation and use of CFD models in the assessment of medical device safety.
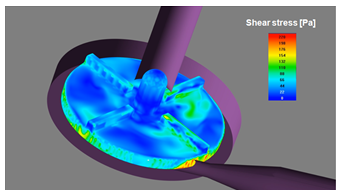
CFD simulations are increasingly being used to determine flow patterns and fluid forces in order to evaluate blood-contacting medical devices. It is due to its potential to calculate the values of physical parameters that may affect the level of blood damage the device may cause, such as shear stress or dwell time. Although CFD can decrease the need for expensive prototyping and laboratory testing, there are no standardized and reliable methods available for using CFD techniques in this field.
The purpose of this project is to determine the limits of the applicability of CFD techniques by comparing some parameters (such as gauge pressure or shear stresses) of the computational simulations of a blood pump in several working conditions against suitable experimental models. The different conditions included a wide range of velocity profiles at the inlet or different rotor velocities.
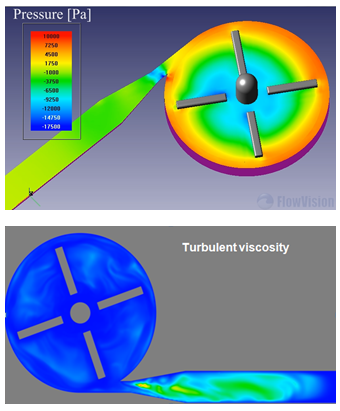
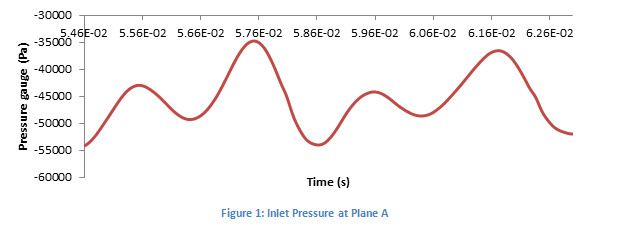
FlowVision moving body capability, together with the real CAD geometry import, has allowed the numerical simulation of this complex case with a relatively simple mesh. Time-dependent results during a whole revolution of the rotor have been obtained, providing consistent and more realistic data for the different scenarios. As example, variables as the pressure gauge behave cyclic with a period of a 1⁄4 of the revolution time, which is consistent with the number of blades.
Images show some physical fields (such as velocity, shear stress, turbulent viscosity and pressure) that are required over different planes and surfaces for all possible cases.